
Assistant Professor &
Ramalingaswami Fellow
- sanjay@cusb.ac.in
- sanjaycdri@gmail.com
- +91- 8287333939
Specialization : Malaria, Cardiovascular, and Blood Cancer(Leukemia
- Malaria research at CSIR-CDRI, Lucknow , U.P, India [2003-2008].
- Cardiovascular research in ion channels, metabolic syndrome, & restenosis in USA [ 2008-2012]
- Blood cancer (Acute leukemia) research in USA [ 2012-2019].
- Teaching experience at Lalit Narayan Mithila University, Darbhanga [ 2019-2020] and at Central University of South Bihar, Gaya [ 2020 – till date].
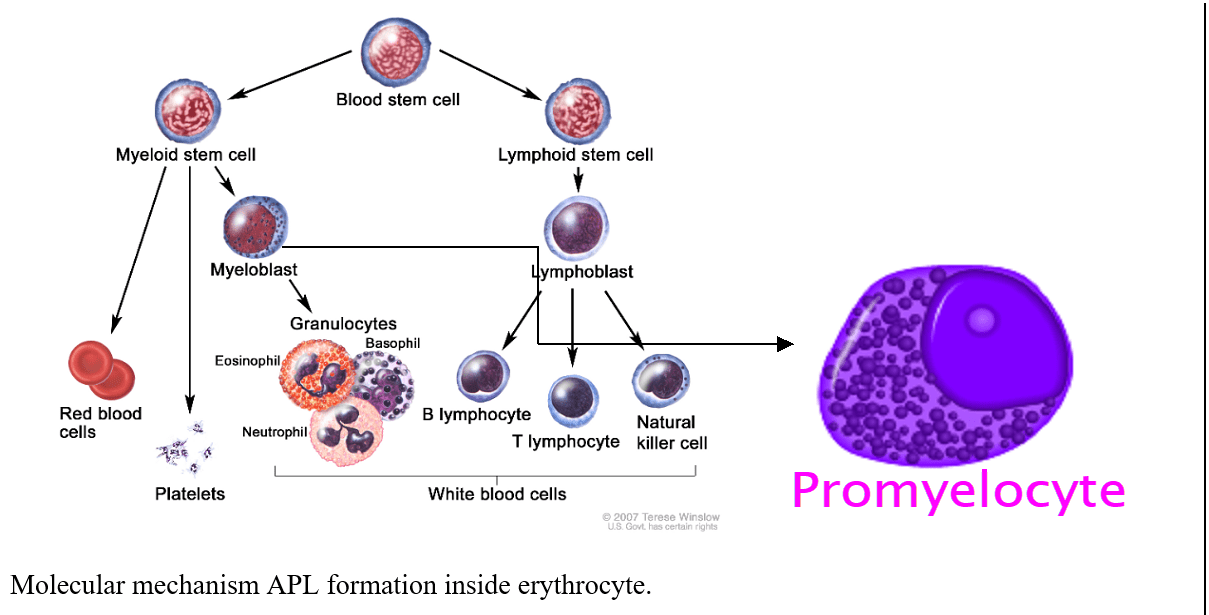
Our malaria research investigates new target or active antimalarial molecules which can be used for new antimalarial drugs designing or novel candidate for vaccine development. We would also study TRPC channels role in metabolic syndrome and signaling mechanism involved in restenosis using human smooth cells /rat model. Acute promyelocytic leukemia [APL] is a blood cancer usually formed due to chromosomal mutation between chromosome 15 and 17. My research aims for identification of novel and new target in APL using APL cell lines, transgenic, and xenograft mice model through utilization of advance molecular, imaging , and RNAi techniques.
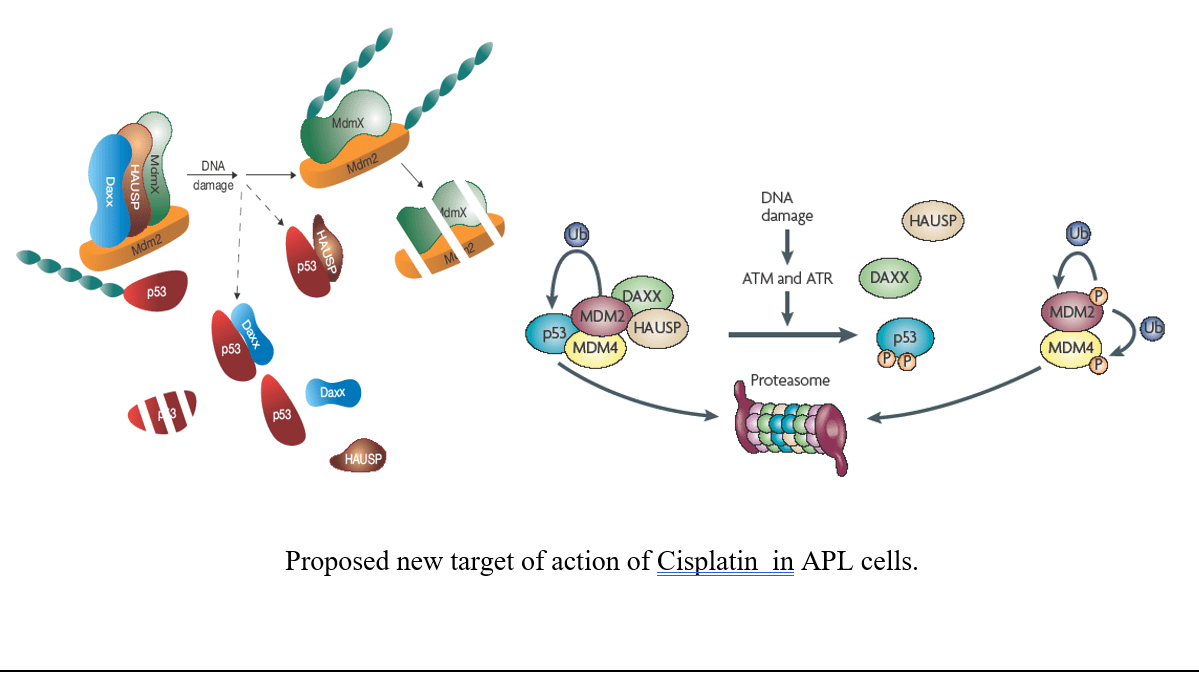
- Publications :
- Kumar S, Tchounwou PB (2022). p53 as a unique target of action of cisplatin in acute leukemia cells. Journal of Cellular and Molecular Medicine (Accepted on 26 th June, 2022).
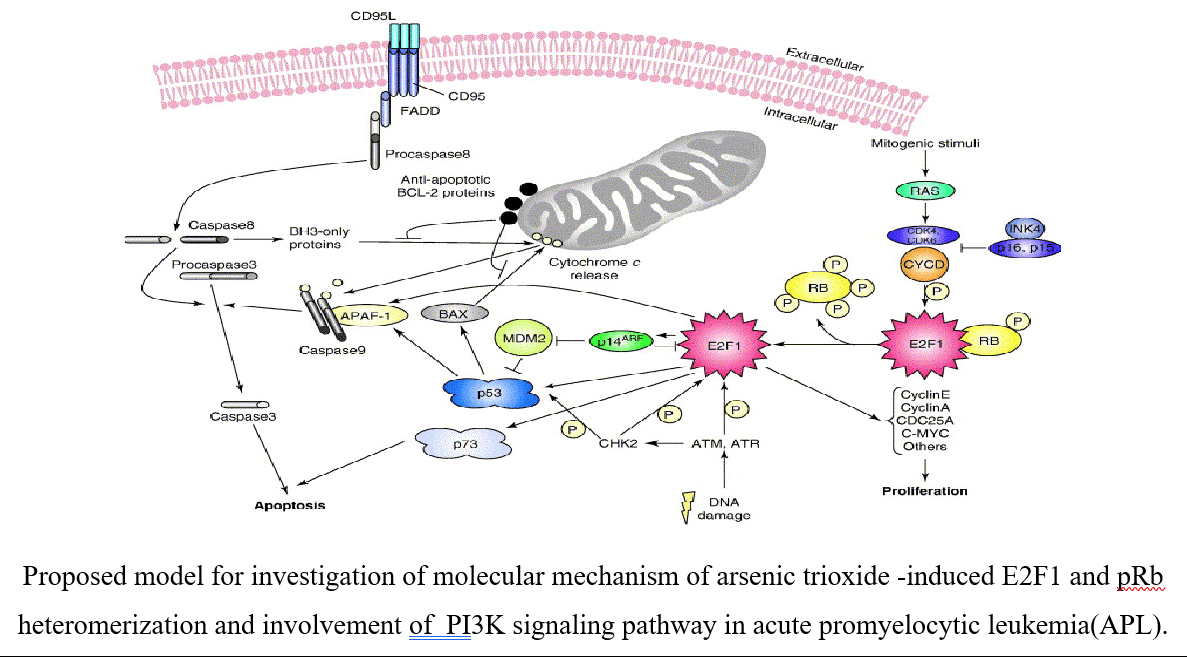
- Sanjay kumar, Paul B. Tchounwou. Arsenic trioxide reduces the expression of E2F1, cyclin E, and phosphorylation of PI3K signaling molecules in acute leukemia cells. Environmental Toxicology 36 (2021), issue 9, 1785-1792. https://doi.org/10.1002/tox.23299
- Paul B. Tchounwou, Shaloam Dasari, Felicite Noubissi, Paresh Ray, Sanjay Kumar. Advances in our understanding of the molecular mechanisms of action of cisplatin in cancer therapy. J. Experimental Pharmacol. 13 (2021), 303-328, doi: 10.2147/JEP.S267383.
- Sanjay kumar, Paul B. Tchounwou. Trisenox disrupts MDM2-DAXX-HAUSP complex and induces apoptosis in a mice model of acute promyelocyte leukemia. J. Cancer, 11 (2020): 4373-4383. doi:10.7150/jca.39996.
- Andrea Brown, Sanjay Kumar, Paul B Tchounwou. Cisplatin-Based Chemotherapy of Human Cancers. J Cancer Sci Ther11(2019):097-103. doi: 10.4172/1948-5956.1000592.
- Paul B. Tchounwou, Clement G. Yedjou, Udensi K. Udensi, Maricica Pacurari, Jacqueline J. Stevens, Anita K. Patlolla, Felicite Noubissi, and Sanjay Kumar. State of the sciencereview of the health effects of inorganic arsenic: perspectives for future research. Environmental Toxicology 34 (2019): 188-202.
- Sanjay kumar, Andrea Brown, Paul B. Tchounwou. Trisenox disrupts MDM2-DAXX- HAUSP complex and activates p53, cell cycle regulation and apoptosis in acute leukemia cells. Oncotarget 9 (2018): 33138-33148.
- Sanjay kumar, Ibrahim O. Farah, Paul B. Tchounwou. Trisenox induces cytotoxicity through phosphorylation of mitogen-activated protein kinase molecules in acute leukemia cells. J Biochem Mol Toxicol. 32 (2018) :e22207.
- Sanjay kumar, Paul B. Tchounwou. Molecular mechanism of cisplatin cytotoxicity in acute leukemia cells. Oncotarget 6 (2015): 40734-40746.
- Sanjay kumar, Clement J. Yedju and Paul B. Tchounwou. Arsenic trioxide induces oxidative stress, DNA damage, and mitochondrial pathway of apoptosis in human leukemia (HL-60) cells. J Exp Clin Cancer Res. 33(2014): 42.
- Venkatesh Kundumani-Sridharan, Nikhlesh K. Singh, Sanjay Kumar and Gadiparthi N.Rao., Nuclear factor of activated T cells c1 mediates p21-activated kinase 1 activation in the modulation of chemokine-induced human aortic smooth muscle cell F-actin stress fiber formation, migration and proliferation and injury-induced vascular wall remodeling. J. Biol. Chem. 288(2013): 22150-22162.
- Nikhlesh K. Singh, Venkatesh Kundumani-Sridharan, Sanjay Kumar, Shailendra K. Verma, Sivareddy Kotla, Hideyuki Mukai, Heckle M.R and Gadiparthi N.Rao.PKN1 is a novel substrate for NFATc1-mediated cyclin D1-CDK6 activity and modulates vascular smooth muscle cell division and migration leading to inward blood vessel wall remodeling. J. Biol. Chem. 287(2012):36291-36304.
- Sanjay Kumar, Saikat Chakraverty, Cindy Balbosa, Tatiana Brustovetsky, NickolayBrustovetsky and Alexander G. Obukhov. Mechanisms controlling neurite outgrowth in Rat pheochromocytoma cell line: The role of TRPC channels. J. Cell. Physiol. 227 (2012):1408-1419.
- Saikat Chakraverty, Zachary C.Berwick, Paula J.Bartlett, Sanjay Kumar, Andrew P. Thomas, Michael Sturek, Jonathan D. Tune, and Alexander G. Obukhov. Bromoenol Lactone (BEL) inhibits Cav1.2, and TRPC channels. J. Pharmacol. Exp. Ther.339 (2011):329-340.
- Zachary P. Neeb, Jason M. Edwards, Mouhamad A. Alloosh, Xin Long, Ian N. Bratz,Cassandra R. Peller, James P. Byrd, Sanjay Kumar, Alexander G. Obukhov, Michael Sturek. Exercise training decreases store-operated Ca2+ entry associated with metabolic syndrome and coronary atherosclerosis. Cardiovasc. Res. 85(2010) 631-640.
- Guoqing Hu, Elena A. Oboukhova, Sanjay Kumar, Michael Sturek, Alexander G. Obukhov. Canonical Transient Receptor Potential Channels Expression is Elevated in a Porcine Model of Metabolic Syndrome. Mol. Endocrinol. 23(2009), 689-699.
- Sanjay Kumar, Mithu Guha, Vinay Choubey, Pallab Maity, Kumkum Srivastava, Sunil K Puri and Uday Bandyopadhyay. Bilirubin inhibits Plasmodium falciparum growth through the generation of reactive oxygen species. Free Rad. Biol. Med. 44 (2008), 602-613.
- Sanjay Kumar, Sajal Kumar Das, Sumanta Dey, Pallab Maity, Mithu Guha, Vinay Choubey, Gautam Panda and Uday Bandyopadhyay. Antiplasmodial Activity of [(Aryl) arylsulfanylmethyl]pyridine.Antimicrob. Agents Chemother. 52 (2008), 705-715.
- Sanjay Kumar, Mithu Guha, Vinay Choubey, Pallab Maity, Uday Bandyopadhyay “Antimalarial drugs inhibiting hemozoin (β hematin) formation: A mechanistic update. Life Sci. 80 (2007), 813-828.
- Sanjay Kumar, Uday Bandyopadhyay “Free heme toxicity and its detoxification systems in human. Toxicol.Letts. 157 (2005), 175-188.
- Mithu Guha, Sanjay Kumar,Vinay Choubey, Pallab Maity, Uday Bandyopadhyay. “Apoptosis in the liver during malaria: Role of oxidative stress and implication of the mitochondrial pathway.” FASEB J. 20 (2006), E439-E449.
- Vinay Choubey, Mithu Guha, Pallab Maity, Sanjay Kumar, Resmi Raghunandan, Prakas R. Maulik, Umesh C. Halder, Uday Bandyopadhyay “Molecular characterization and localization of Plasmodium falciparum choline kinase.” Biochim Biophys Acta. 1760 (2006), 1027-1038.
- Mithu Guha, Vinay Choubey, Pallab Maity, Sanjay Kumar, Kumkum Shrivastava, Sunil K. Puri and Uday Bandyopadhyay. Overexpression, purification and localization of apoptosis related protein from Plasmodium falciparum. Protein Expr.Purif. 52 (2007), 363-372.
- Vinay Choubey, Pallab Maity, Mithu Guha, Sanjay Kumar, Kumkum Shrivastava, Sunil Kumar Puri and Uday Bandyopadhyay. Inhibition of Plasmodium falciparum choline kinase by hexadecyltrimethylammonium bromide: A possible antimalarial mechanism of hexadecyltrimethylammonium bromide. Antimicrob. Agents Chemother. 51 (2007), 696-706.
- Tchounwou PB, Udensi KU, Isokpehi RD, Yedjou CG, Kumar S.(2015). Arsenic and cancer. Chapter 23 in Handbook of Arsenic Toxicology. Flora SJS (Ed). Elsevier Inc. London. pp 533-555.
- Awards :
- Editorial board member of GERF Bulletin of Bioscience. http://www.gerfbb.com/editorialboard.php
- Reviewer of four international journals: - (i) Circulation, ii) Circulation Research, (iii) Life Sciences, (iv) Environmental Toxicology.
- University Grant Commission (UGC) start up grant (2021-23), Department of Science and Technology, Govt. Of India.
- Ramalingaswami Re-entry Fellowship [2019], Department of Biotechnology, Govt. Of India.
- Presidential Creative Award (2015) [Jackson State University, Jackson, MS, USA].
- Research Excellence Award (2014) [Research Centers in Minority Institutions (RCMI), USA].
- Junior Research Fellow[JRF] and Senior Research Fellow[SRF] (2003-2007) [Council of Scientific and Industrial Research (CSIR), India].
- Projects:
- UGC-BSR research Start-Up Grant from University Grant Commission, Govt. of India (2021-22), (10 Lakh).
- Ramalingaswami Re-entry Fellowship [2019], Department of Biotechnology, Govt. Of India. (2019-2024) {118 lakh}.
- Intermediate fellowship from India Alliance and Wellcome trust 1st step completed and full application under process
-
- Oral Presentation
- Kumar S. Mode of action of arsenic trioxide in mice model of acute leukemia.16th International Symposium on Metal Ions in Biology and Medicine [30th November – 1st December, 2021) at Nehru Science Centre, Mumbai, India. Abstract No. O-24.
- Kumar S. Trisenox inhibits growth of blood cancer cells through regulatorn of E2F1 and pRb expression. International Conference on “Innovative Research in Life Science for Substainable Development”. [29-30 July, 2021] at Department of Botany of LNMU, Darbhanga, India.
- Kumar S. COVID and mucormycosis: partners in crime. First International Online Conference post Covid-19 associated Black & White fungal infection. [14th June, 2021] at Department of Biosciences, School of Basic sciences, Faculty of Science and Directorate of Admissions, Manipal University, Jaipur, India.
- Kumar S. Health for all. One day international web conference and scientific seminar. [30th June, 2020] at Department of Botany, T.P.S College, Patna, India (online mode).
- Kumar S. Novel mode of action of cisplatin in acute leukemia cells. 8th Bihar Science Conference [26-28th. March, 2020] at Campus of Patna University, Bihar, India. (online).
- Kumar S. Possible role of genetic engineering in bamboo tree improvement. National seminar –cum-training programme on propagation, management and value addition of baboo plants for socio-economic development[ 26-27th . February, 2020] at B.S.S College Supaul -852131, Bihar, India.
- Kumar S and Tchounwou PB. Trisenox reduces expression of E2F1, cyclin E, and phosphorylation of PI3K signaling in APL cells. 15th International Symposium on Recent Advances in Environmental Health Research (17-20th February, 2019) at Marriott hotel, Jackson, MS, USA, Abstract No. O-08.
- Kumar S and Tchounwou PB. Trisenox induces cell cycle regulation and apoptosis through modulation of MAPK pathway in acute leukemia cells . 33rd Annual meeting southern biomedical engineering conference (17-19th March, 2017) at Marriott beachfront, Gulfport, MS, USA, Abstract No. O-49.
- Kumar S and Tchounwou PB. Arsenic trioxide causes cell death through disruption of MDM2-DAXX-HAUSP complex and increased promyelocytes formation in mouse model of acute promyelocytic leukemia. 12th International Symposium on Recent Advances in Environmental Health Research (13-16th September, 2015) at Marriott hotel, Jackson, MS, USA, Abstract No. O-34.
- Kumar S and Tchounwou PB. Trisenox disrupts MDM2-DAXX-HAUSP complex and degradation of MDM2 in acute promyelocytic leukemia cells. International conference on clinical trials (27-29th July, 2015) at Hyatt hotel, Orland, FL, USA.
- Kumar S and Tchounwou PB. Cisplatin induces oxidative stress and cytotoxicity, and modulates stress signaling in human leukemia (HL-60) cells. 11th International Symposium on Recent Advances in Environmental Health Research & 13th international symposium on metal ions in biology (14-18th September 2014) at Jackson Convention Centre, Jackson, MS, USA, Abstract No. O-41.
- Kumar S, Yedjou CG., Tchounwou PB. Arsenic trioxide causes cell cycle arrest and induces intrinsic pathway of apoptosis in human leukemia (HL-60) cells. 10th International Symposium on Recent Advances in Environmental Health Research (15- 18th September 2013) at Cooking Convention Centre, Jackson, MS, USA, Abstract No. O-05.
- Kumar S, Guha M., Puri SK., Bandyopadhyay U. Bilirubin induces apoptosis like death in Plasmodium falciparum through the augmentation of oxidative stress. Society for Free Radical Research - India [SFRR –India] Satellite Meeting 7-9 February, 2008. AIIMS, New Delhi, India. Abstract No. O- 14.
- Kumar S., Guha M., Puri SK. Bandyopadhyay U., Bilirubin causes apoptosis in P.falciparum through oxidative stress through generating reactive oxygen species. 19th National Congress of Parasitology and International Symposium on Parasitic Diseases of Animals & Man. 22-24 October, 2007, Andhra University, Visakhapatnam, India. Abstract No. O- 24.
- Poster Presentations
- Paul Tchounwou Sanjay Kumar, Molecular mechanism of cisplatin-induced p53 activation, cell cycle regulation, and apoptosis in acute leukemia cells. Annual virtual Meeting of the American Association for Cancer Research; April, 8-13th, 2022, New Orleans, LA; USA.
- Sanjay Kumar, Paul Tchounwou. Arsenic trioxide reduces the expression of E2F1,cyclin E , and phosphorylation of PI3K signaling molecules in acute leukemia cells. Annual virtual Meeting of the American Association for Cancer Research; April, 10-15th, 2021; USA.
- Paul B. Tchounwou, Sanjay Kumar. Cisplatin disrupts MDM2-DAXX-HAUSP complex, & induces apoptosis in acute leukemia cells . Proceedings of the Annual Meeting of the American Association for Cancer Research 2020; 2020 Apr 27-28 and Jun 22-24. Philadelphia (PA): AACR; Cancer Res 2020;80(16 Suppl):Abstract nr 606.
- Sanjay Kumar, Paul Tchounwou. Cisplatin disrupts MDM2-DAXX-HAUSP complex, p53 accumulation, cell cycle regulation, and apoptosis in acute leukemia cells. 110 th Annual Meeting of the American Association for Cancer Research; March 29- 3rd April, 2019; Atlanta, USA, Abstract No. 5239.
- Sanjay Kumar, Paul Tchounwou. Arsenic trioxide inhibits proliferation of acute leukemia cells through reduced expression of E2F1, cyclin E, and activation of retinoblastoma protein. 109th Annual Meeting of the American Association for Cancer Research; Apr 14-18, 2018; Chicago, IL, USA, Abstract No. 1503.
- Tchounwou PB, Kumar S. Molecular mechanisms of cisplatin cytotoxicity in APL cells. RCMI translational science 2017 (Oct 28-1st Nov.1, 2017) Marriott Wardman Park hotel, Washington, DC, USA, Abstract No. 01.02.004.
- Sanjay Kumar, Paul Tchounwou. Cisplatin disrupts MDM2-DAXX-HAUSP complex, MDM2 degradation, and activation of p53 in murine model of APL. 14th International Symposium on Recent Advances in Environmental Health Research (11-13th September 2017) at Marriott Hotel, Jackson, MS, USA, Abstract No. PB-04.
- Andrea Brown, Sanjay Kumar, Paul Tchounwou. Cisplatin induces reactive oxygen species, changed gluthione levels, and activates apoptosis in human acute leukemia cells. 14th International Symposium on Recent Advances in Environmental Health Research (11-13th September 2017) at Marriott Hotel, Jackson, MS, USA, Abstract No. PA-01.
- Sanjay Kumar, Paul Tchounwou. Arsenic trioxide modulates P38MAPK pathway of signaling in acute promyelocytic leukemia. 13th International Symposium on Recent Advances in Environmental Health Research (11-14th September 2016) at Marriott Hotel, Jackson, MS, USA, Abstract No. PA-07.
- Sanjay Kumar, Paul Tchounwou. TRISENOX disrupts MDM2-DAXX-HAUSP interaction and increased promyelocytes formation in murine model of APL. In: Proceedings of the 107th Annual Meeting of the American Association for Cancer Research; 2016 Apr 16-20; New Orleans, LA. Philadelphia (PA): AACR; Cancer Res 2016;76(14 Suppl): Abstract nr 3704.
- Sanjay Kumar, Paul B. Tchounwou. Arsenic trioxide induces cell cycle arrest, apoptosis through interaction of DAXX and degradation of MDM2 in acute leukemia cells. In: Proceedings of the 106th Annual Meeting of the American Association for Cancer Research; 2015 Apr 18-22; Philadelphia, PA. Philadelphia (PA): AACR; Cancer Res 2015; 75(15 Suppl): Abstract nr 3811. doi:10.1158/1538-7445.AM2015-3811.
- Kumar S., Yedjou CG., Tchounwou PB. Trisenox, cell cycle arrest, MAPK signaling and apoptosis in HL-60 cells. 2014 Minority Health and Health Disparities Grantees’ Conference (1-3rd December, 2014) National Harbor convention Centre, MD, USA, Abstract No. 01.02.01.057.
- Sanjay Kumar, Clement Yedjou, Paul B. Tchounwou. Arsenic trioxide induces cell cycle arrest, apoptosis and MAPKinase signaling cascade in acute promyelocytic leukemia cells. In: Proceedings of the 105th Annual Meeting of the American Association for Cancer Research; 2014 Apr 5-9; San Diego, CA. Philadelphia (PA): AACR; Cancer Res 2014;74(19 Suppl): Abstract nr 2291. doi:10.1158/1538-7445.AM2014-2291.
- Njiki S, Kumar S., Kafoury R., Begonia G., Yedjou CG. Garlic Extract Modulated Phosphatidylserine Externalization Accompanied by Secondary Necrotic Cell Death in Leukemic Cells. 10th International Symposium on Recent Advances in Environmental Health Research (15-18th September 2013) at Cooking Convention Centre, Jackson, MS, USA, Abstract No. PA-08.
- Kumar S., Yedjou CG., Tchounwou PB., Arsenic Trioxide Induced Transcriptional Activation of Stress Genes and Program Cell Death of Human Leukemia Cells. 9th International Symposium on Recent Advances in Environmental Health Research (16-19th September 2012) at Marriott Hotel, Jackson, MS, USA, Abstract No. PB-12.
- Kumar S., Nunez CMB., Chakraborty S., Obukhov AG. The role of TRPC channels in regulating the length of neurite. Annual IUSM and Clinical and Translational Sciences Institute (CTSI) Scientific Poster Session, 16th September, 2009 at School of Medicine Venue, IUPUI, Indianapolis, IN, USA. Abstract No. P-10.
- Kumar S., Guha M., Choubey V., Bandyopadhyay U. Molecular characterization of a putative gene from the human malaria parasite, Plasmodium falciparum. International Symposium on Chemical Biology held on 7-9th March, 2007 at Indian Institute of Chemical Biology, Kolkata, India. Abstract No. P-15.
- Oral Presentation
-
-
- Life member of the Indian society of parasitology
- Associate Member of American Association of Cancer Research
-
-
-
- Assistant Professor in Department of Life Sciences, School of Earth, Biological, and Environmental Sciences, Central University South Bihar, Gaya, India (16th June 2020 – continue) [https://www.cusb.ac.in/index.php/110-faculty-profile/life-science ]
- Assistant Professor in University PG Department of Botany, Lalit Narayan Mithila University [LNMU], Darbhanga, India (2nd September 2019 – 15th June 2020).
- Co-ordinator, University PG Department of Biotechnology, Lalit Narayan Mithila University [LNMU], Darbhanga, India (January 2020- 15th June 2020).
- Ramalingaswami Fellow, Department of Biotechnology, Govt. of India [ November 2019 - continue].
- Postdoctoral Research associate, Jackson state university, Jackson, MS USA, (16th April, 2012- 1st September 2019) [ https://www.jsums.edu/science/2014/12/17/dr-sanjay-kumar- award/ ].
- Postdoctoral Fellow, University of Tennessee Health Science Centre, Memphis [UTHSC], TN USA, (1September 2010 – 15th April 2012).
- Postdoctoral Fellow, Indiana University, Indianapolis IN USA, (1st March 2008 –31st August 2010).
- Research Scholar, CSIR- CDRI Lucknow, India (23rd February 2003- 28th February 2008)
-
- Postdoctoral
- Graduate (Ph.D)
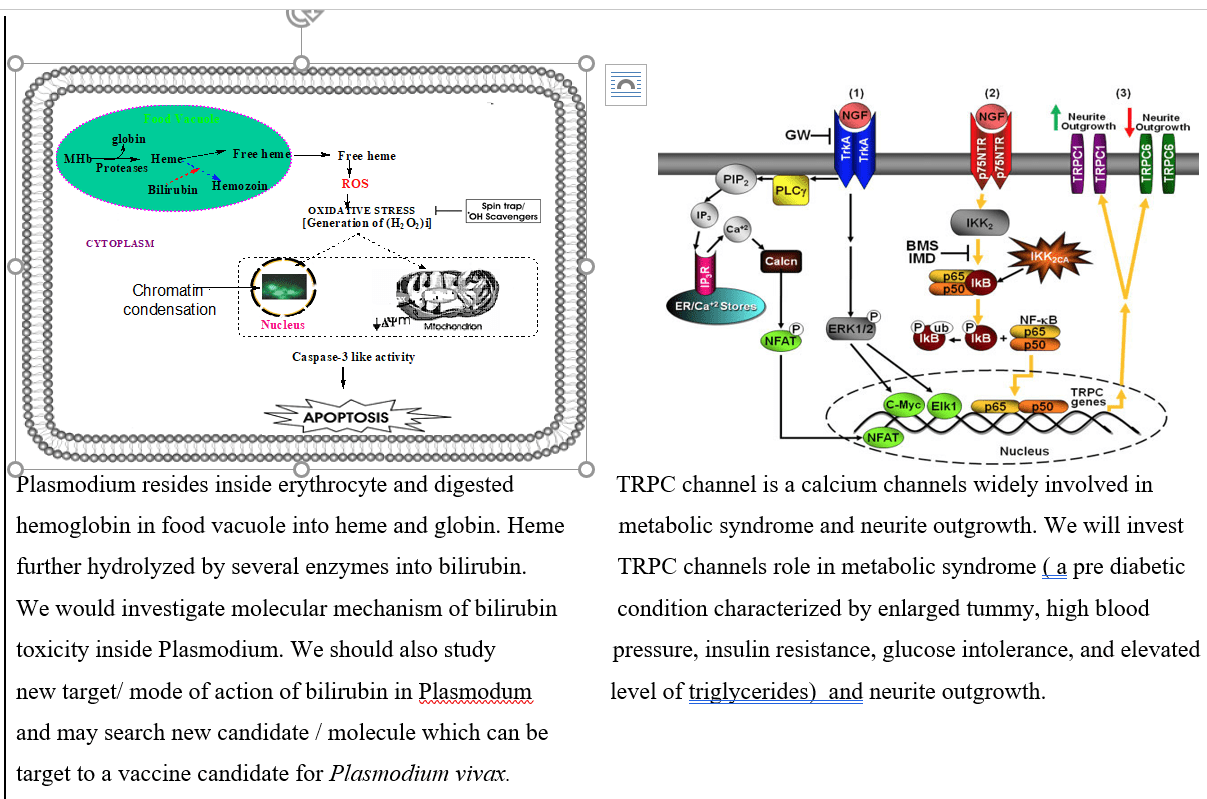
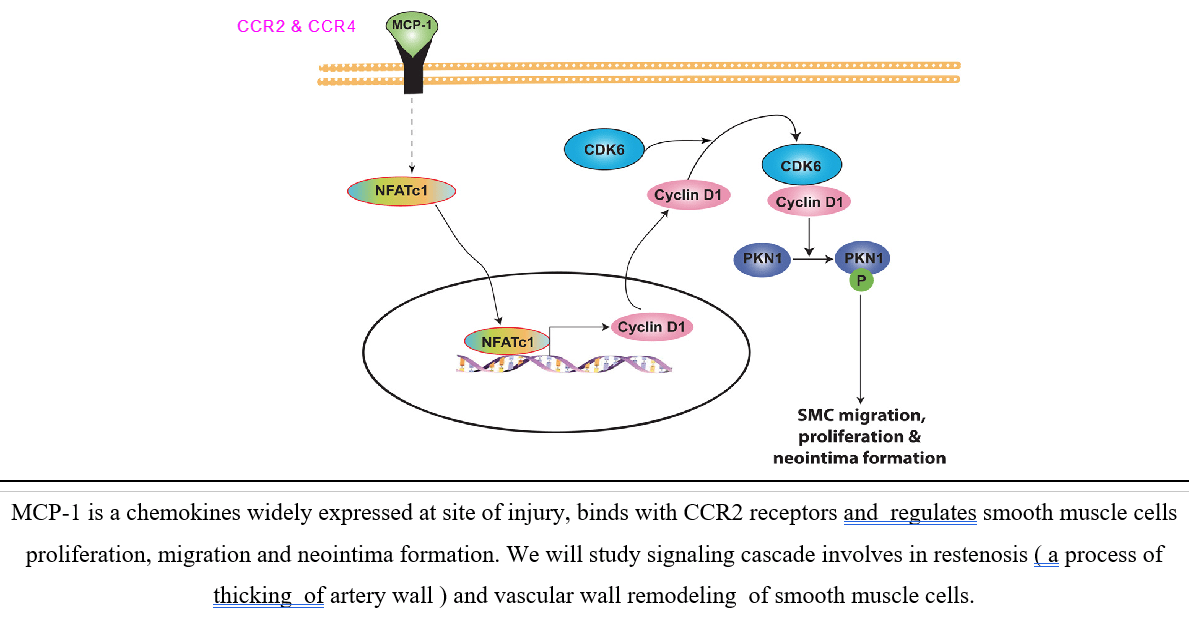
My research works were investigated new target of action of arsenic trioxide (ATO) and cisplatin (CDDP) in both acute promyelocytic leukemia (APL) cell lines & transgenic mice. We identified MDM2-DAXX-HAUSP complex in APL and checked effect of both ATO & CDDP on complex molecules expression, association, and p53 activation simultaneously. We also investigated p53 role in APL cell cycle regulation and apoptosis. We were investigated monocyte chemotactic protein (MCP-1) role in NFATs mediated restenosis using human aortic smooth muscles cells (HASM) and rat carotid artery as model to revealed signaling mechanism involved in smooth muscles cells growth, motility and restenosis. We were applied varieties of molecular biology tools like cell migration, tube formation, DNA synthesis, balloon injury induced rat carotid artery (in vivo) model for investigation of MCP-1 role in smooth muscles migration, proliferation and neointima formation. Dr. Obukhov laboratory projects were related to investigation of TRPC channel role in metabolic syndrome and neurite outgrowth. It was involved cloning, sub cloning of different subfamilies of TRPC channel of pig, site directed mutagenesis, transfection, Ca2+ measurement (by Fura-2 loading), localization by confocal microscopy and functional characterization of channels by electro-physiological and Co-IP approach. We identified splice variants of TRPC6 channel in pig and cultured human coronary arteries smooth muscles cells and characterized by different molecular, biochemical and electrophysiological approach. We also investigating TRPC channel role in regulation of NGF - induced neurite outgrowth in PC12 cell line as well as hippocampal neuron.
I was completed three projects in Dr. Bandyopadhyay laboratory at CDRI, Lucknow, India. First project was involved cloning of Plasmodium falciparum hypothetical genes, over expression, localization and characterization of their recombinant proteins. Another project was related to investigation of molecular mechanism of free heme toxicity and antimalarial molecules that enhance free heme toxicity in Plasmodium falciparum. Last project was related to study of hepatocyte/liver apoptosis during malaria infection in mice.
Plasmodium falciparum genes cloning, over expression, localization and characterization of their recombinant proteins
We were cloned four Plasmodium falciparum hypothetical genes like geranylgeranyltransferase, heme binding protein, hemolysin and apoptosis related protein. Clones were transformed in Rosetta vectors (expression vector) and purified over expressed recombinant proteins. Recombinant proteins were localized in Plasmodium falciparum by western blotting, confocal and fluorescence microscopy through leveling different dyes. We were also characterized above over expressed recombinant proteins by CD spectra analysis, Km value, native and subunit mass (using HPLC, MALDI-TOF) determination, molecular modeling, stage specific expression and enzyme kinetics.
Investigation of molecular mechanism of free heme toxicity
Free heme is very toxic to malarial parasite because it kills parasite with unknown mechanism. We were investigated first time detail molecular mechanism of free heme (hemin) toxicity in malaria parasite. We found, hemin induced oxidative stress through generating hydroxy radicals (.OH) in parasite via intermediate formation of H2O2 through lipid peroxidation, protein carbonyl formation in membrane and genomic DNA damage. I was also screened several active anti- malarial molecules that acted through enhancing free heme concentration by preventing heme polymerization into hemozoin in parasite both in vitro using cultured Plasmodium falciparum, as well as in vivo murine model (P. yoelii) of parasite. Among active antimalarial molecules, [(Aryl) arylsulfanylmethyl] pyridine (AASMP) and bilirubin prevent hemozoin formation in parasite and increased free heme concentration to kill the parasite. We were done pharmacological scavenger or radical trap of hydroxyl radical (e.g. PBN, TEMPO) studied to confirm that hydroxyl radical generation from free heme using parasite lysate with or without AASMP treated P. falciparum culture. We discovered bilirubin -induced oxidative stress and apoptosis like death of Plasmodium falciparum through changing mitochondrial membrane potential and stimulating caspase-3 activity as well as expression of putative apoptosis related protein (PfARP).
Host parasite interactionThis project was related to investigation of effect of malaria infection in host liver. Liver dysfunction is common feature during malarial infection in the host, which often leads to various clinical complications such as jaundice and hepatomegaly. While investigating the route of this liver dysfunction, we were investigated mice liver undergo intrinsic pathway of apoptosis during malaria infection.
TEACHING AND MENTORING EXPERIENCEI am teaching genetics, animal behavior, parasitology, plant biotechnology, molecular & cell biology, Plant pathology, animal diversity, recombinant DNA technology & tissue culture, and cytogenetics of both graduate and master students. I have been providing research guidance /mentoring several graduate and master students, technicians, and PhD scholar several years.
- 2008-10 Cindy M. Barbosa, T32 student,
- 2013-14 Tylen Miller, undergraduate student
- 2012-14 Christian Roger, Lab technician.
- 2016-18 Andria Brown, Graduate student
- 2020-21: Gopi Krishna Das [ M.Sc. in Life sciences, dissertation student]
- 2020-21: Aditi [ M.Sc. in Life sciences, dissertation student]
- 2020-21: Pesalika Sahu [ M.Sc. in Life sciences, dissertation student]
- 2020-21 Smrutirekha Samantaray [M.Sc. in Life sciences, dissertation student]
- 2020-21: Jyotshna Mayee Beg [M.Sc. in Life sciences, dissertation student]
- 2021-22: Swati Banerjee [M.Sc. in Life sciences, dissertation student]
- 2021-22: Swati smriti Dhan [M.Sc. in Life sciences, dissertation student]
- 2021-22: M.K Tiwari [M.Sc. in Life sciences, dissertation student].
- 2021-25: Priya Kumari [PhD Scholar].
- 2021-25: Ananta [PhD Scholar].
See below few of my students and technicians name below:











